Review of Basic Electrostatics
Part 1
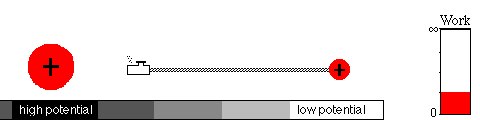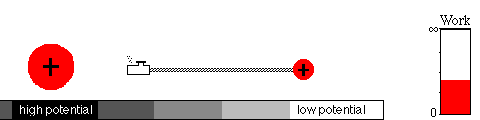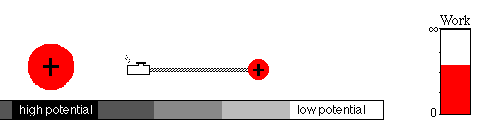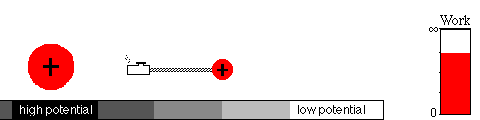
A battery works by means of a slow chemical reaction. Two ionic
chemicals, with different potentials, exchange ions (charged particles)
and allow current to flow between the terminals of the battery.
Eventually, the ions will have moved to the region of lower potential,
equalizing the potential of the two chemicals. That's what happens when
your battery goes dead.
What a battery recharger does is to create an artificial region of
potential, using the electric current in your outlet. This region of
potential reverses the flow of ions from one chemical to the other,
forcing them into their original configuration. It acts like the motor in
the picture above, bringing bringing ions back together so that you can
use the battery again.
If you have not yet read the Second Part
of the Review, you should do so now. Otherwise, go on to learn the
Definition of Potential
Copyright
1998-2000
Rensselaer Polytechnic Institute. All Rights Reserved.

